
In light of the coronavirus (Covid-19) outbreak, GlobalData has analysed the five largest Covid-19 clinical trials in terms of participant size.
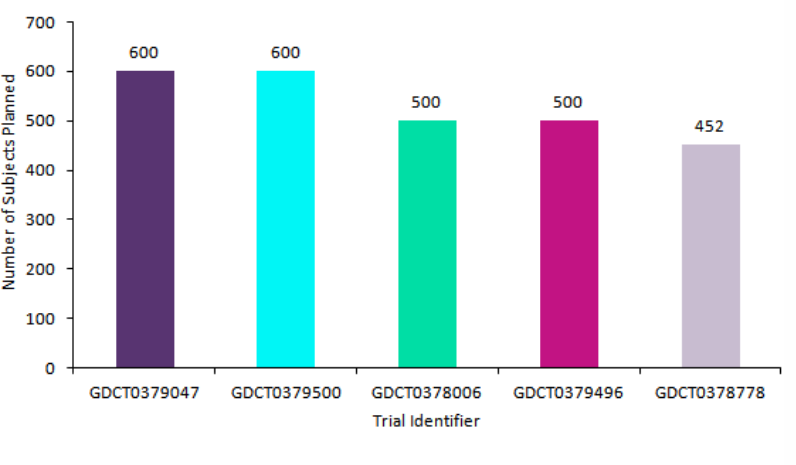
GDCT0379047 (ChiCTR2000029573) and GDCT0379500 (ChiCTR2000029602) are the two largest coronavirus trials, with both trials aiming to recruit 600 participants. Trial GDCT0379047, an interventional planned Phase IV trial, tests the efficacy and safety of a recombinant cytokine gene-derived protein injection combined with Umifenovir (Arbidol), Lopinavir / Litonavir. The study aims to combine the immunostimulatory effects of the cytokine gene-derived protein injection with the anti-viral properties of umifenovir, Lopinavir / Litonavir against Covid-19. This trial is located in Zhejiang, China and is sponsored by the Zhejiang University School of Medicine Hospital 1 in collaboration with Jiehua Biotechnology (Qingdao) Co Ltd.
Trial GDCT0379500, an interventional ongoing Phase 0 study, aims to test the preventative effects of traditional Chinese medicine (TCM) against Covid-19. This study aims to determine if the addition of TCM to standard health education is more effective than health education alone in preventing Covid-19. This trial is located in Hubei, China and is sponsored by the Hubei Hospital of Traditional Chinese Medicine in collaboration with the State Administration of Traditional Chinese Medicine.
Furthermore, trial GDCT0378006 (NCT04246242), an interventional planned Phase IV trial, aims to recruit 500 patients to investigate the use of umifenovir as an add-on to antiviral combination therapy. The trial is split into three arms and conventional antiviral therapy is utilized in the control arm. The two experimental arms study the administration of 200mg and 400mg of umifenovir, respectively, alongside conventional antiviral therapy in the treatment of coronavirus. This trial is located in China and is sponsored by Xiangya Hospital of Central South University.
Trial GDCT0379496 (ChiCTR2000029624), alongside the previously mentioned GDCT0379500, analyzes the use of TCM; however, trial GDCT0379496 is studying TCM as a treatment as opposed to a preventative measure. There is also a variation in study type, as GDCT0379496 is an observational planned Phase IV study, while GDCT0379500 is an interventional trial. In study GDCT0379496, 500 coronavirus patients who received TCM as a treatment will be assessed to optimize the diagnosis and treatment program. This trial is located in Shanghai, China and is sponsored by Shanghai Public Health Clinical Center in collaboration with the Shanghai Municipal Health and Family Planning Commission.
Trial GDCT0378778 (NCT04257656), an interventional ongoing Phase III trial, is utilizing remdesivir, an anti-viral medication which is in development and indicated for Ebola virus, as a treatment Coronavirus. Remdesivir acts by blocking the viral RNA replication process, thereby preventing viral propagation and leading to a decrease in disease pathogenicity. This trial aims to recruit 452 subjects and serves as the only study within the top five that does not utilize umifenovir or TCM. The trial is located in Beijing, China and is sponsored by Capital Medical University.
Evidently, a substantial research effort has been geared toward therapies utilizing umifenovir, or TCM with four of the top five clinical trials involving these agents. Owing to the public health emergency caused by the coronavirus, the interim results of these trials will yield important results to address the urgent need for effective therapies. Nevertheless, to effectively combat the disease a greater level research and collaboration is required worldwide; currently, an overwhelming amount of the research is being conducted in China, which may limit the scope of potential therapies being investigated. China Evergrande group, a real estate company listed on Fortune Global 500, aims to improve collaborative efforts by awarding a joint $115M grant to Harvard and Guangzhou Institute of Respiratory Disease, with the principle aim of enhancing diagnostic methods and treatment options of Covid-19 respiratory disease.